How Many Electrons Are Shared in a Double Covalent Bond
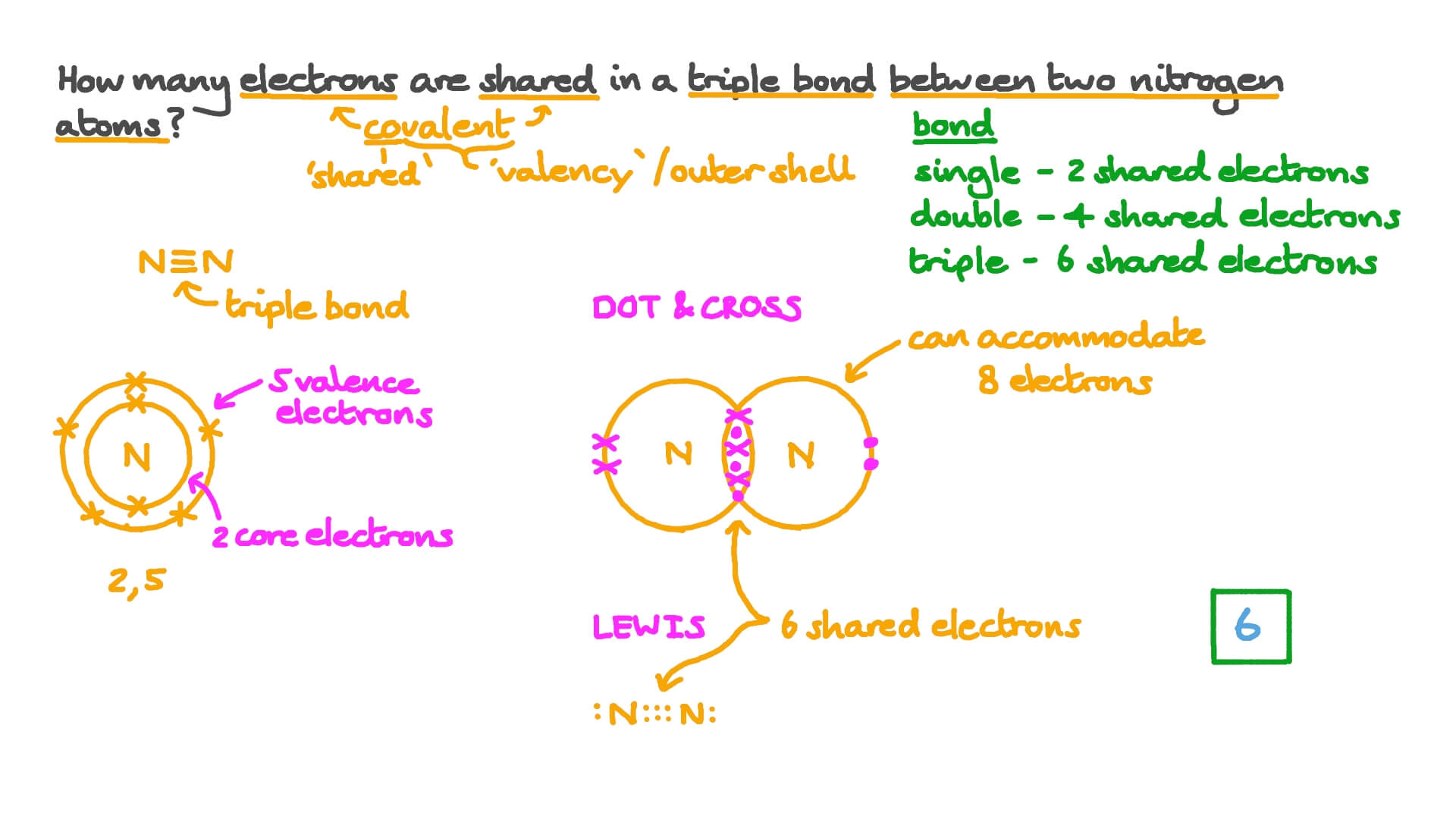
In HCN Carbon is bonded to Nitrogen with a triple covalent bond consisting of one sigma bond and two pi bonds. And since atoms are by definition neutral we have eight electrons as well eight electrons to balance out the charge of the eight protons.
1 Covalent Bonding L L Takes Place Between
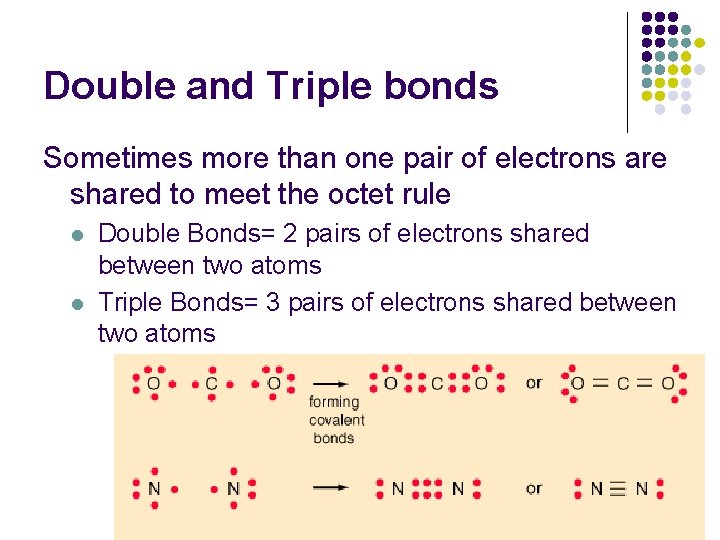
That means that a double bond will have 4 electrons in the space between the bonded nuclei and a triple bond will have 6.
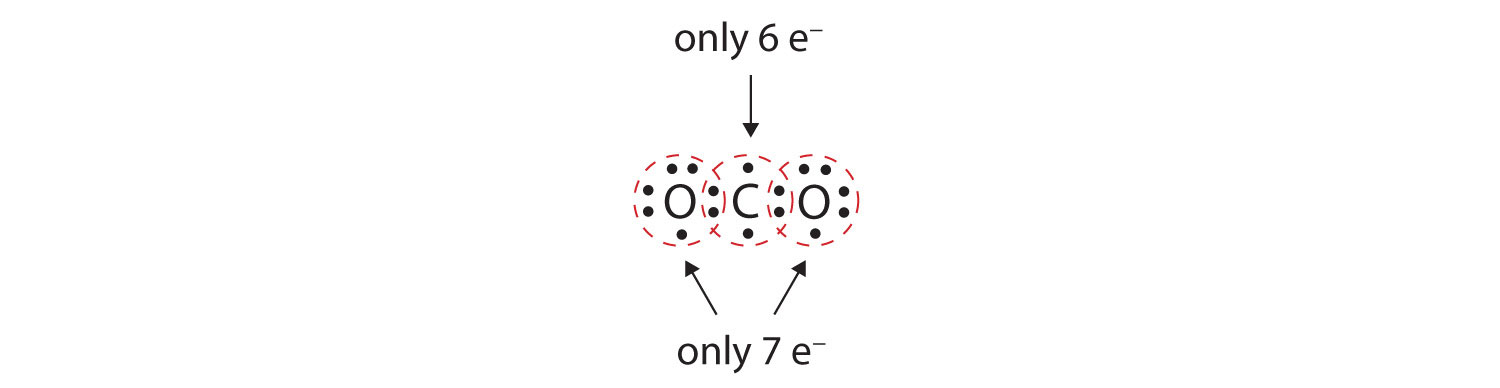
. 100 4 ratings In a double covalent. CO_2 has the carbon in the middle and a double bond to. Molecular is the same as _________.
How many electrons are shared in each. A single covalent bond is formed by sharing one pair of electrons between atoms. Thus a double covalent bond results from the sharing of 4 electrons together by two atoms.
Half the internuclear distance between two atoms bonded by a single covalent bond. View the full answer. Number of electrons shared in double covalent bond is 4.
4 2 See answers Advertisement Advertisement schoolthingz schoolthingz Answer. D in a single covalent bond. Thus a single covalent bond is formed when atoms share 2 electrons together.
Examples of compounds with double bonds include oxygen gas carbon dioxide acetone and ozone. Covalent bonds can be single double or triple covalent bonds. In a double covalnet bond four electrons are shared.
There exist very powerful chemical bonds between atoms. A triple bond is formed by the sharing of three pairs of electrons between two. Each atom contributes one electron.
How many pairs of electrons are shared in a double bond. The double covalent bond is formed when each participating atom contributes two electrons each to be equally shared between them in order to form a double covalent bond. Wiki User 2011-01-09 003005.
The electrons involved are in the outer shells of the atoms. The question asks about electrons shared in a double bond between two oxygen atoms. A double covalent bond is the type of chemical bond in which two electron pairs are shared between the two atoms.
This means that oxygen atoms contain eight protons. Valence Shell Electron Pair Repulsion- repulsion between the electron pairs causes the molecule shape to adjust so the electrons stay as far apart as possible. Bond Strength Chemistry LibreTexts.
How many electrons do two atoms in a double covalent bond share. 2 What type of bond is formed when electrons are not equally shared between two atoms. Formation of oxygen molecule.
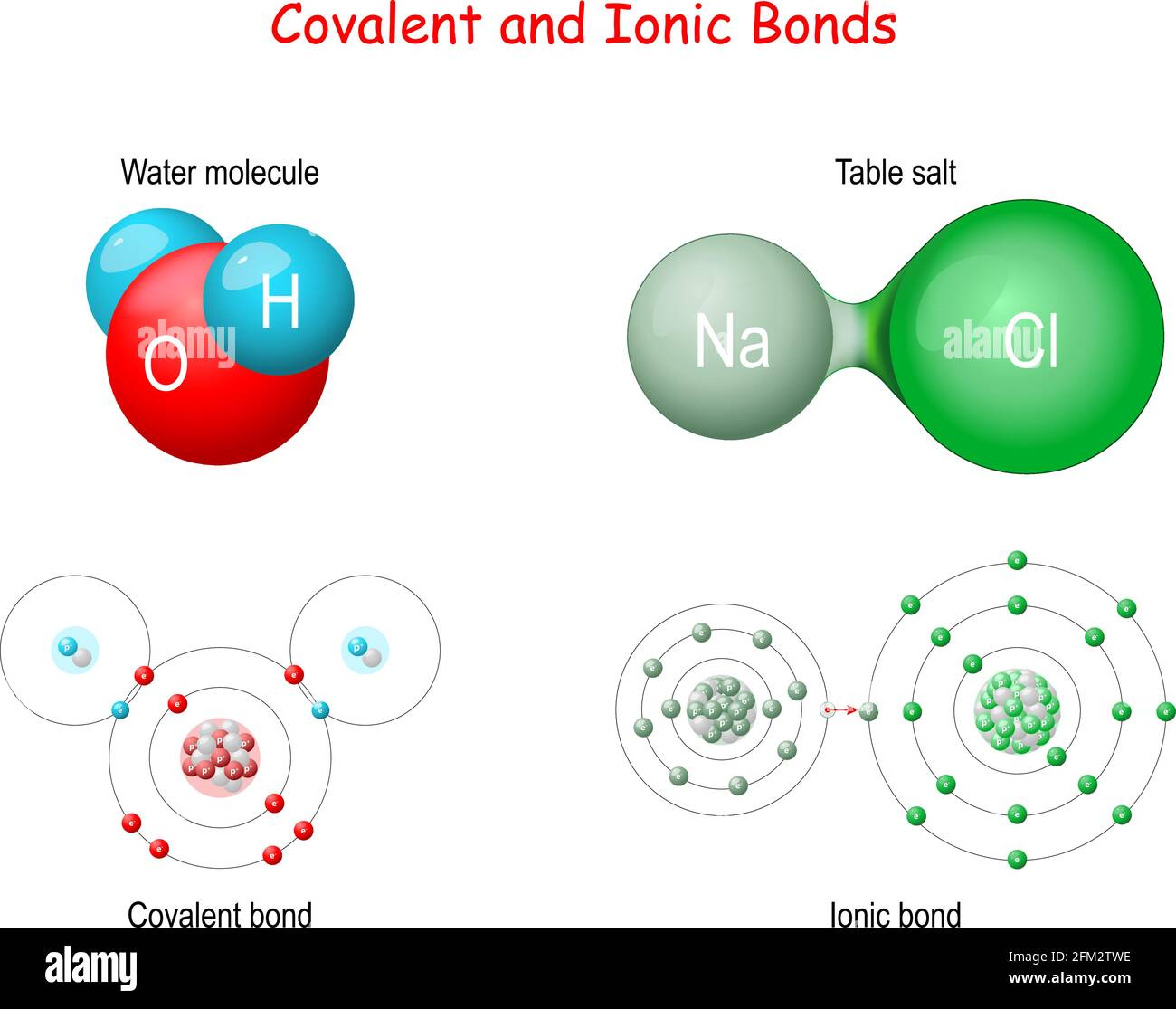
See the answer See the answer done loading. Comparable is the case with C It wants 4 extra electrons for which it tends to kind both 4 single bonds 2 double bonds or one single and one triple bond. A covalent bond forms when two non-metal atoms share a pair of electrons.
The sharing of two electrons is known as a covalent bond. A covalent bond in which one pair of electrons is shared. Thus a double covalent bond results from the sharing of 4 electrons together by two atoms.
C When melted they conduct an electric current. The nuclei of two attached atoms. The shape of a molecule that has three single covalent bonds and one lone pair on the central atom is.
Click hereto get an answer to your question How many electrons are shared in a double covalent bond. Ionic compounds are usually soluble in polar substances. The electrons involved are in the outer shells of the atoms.
An atom that shares one or more of its. How many electrons are shared in a double covalent bond. Give an example of each type.
A double covalent bond is where two pairs of electrons are shared between the atoms rather than just. A double bond is formed by sharing two pairs of electrons between two atoms. Most covalent bonds involve the sharing of ------- electrons.
In ______ bonding the valence electrons are shared among all the atoms of the metallic elements. How many pi bonds are in HCN. Number of electrons shared in double covalent bond is 4.
4 When atoms share electrons. How many in a. Correct option is.
Double covalent bonds are considered reactive bonds because of the presence of. Covalent bonding does not result in the formation of new electrons. Number of electrons shared in double covalent bond is 4.
A triple bond is formed by the sharing of three pairs of electrons between two. Covalent bonds include single double or triple bonds where 2 4 or 6 electrons are shared respectively. D They are composed of metallic and nonmetallic elements.
How many electrons are shared in a double covalent bond. B They have low melting points. A They are solids.
How many electrons are shared in a double covalent bond. A covalent bond in which two pairs of electrons are shared. This is the best answer based on feedback and ratings.
A covalent bond normally contains the energy of about 80 kilocalories per mole kcalmol. The atomic number of the element oxygen is eight. 3 When bonding electrons are shared unequally the bond that is formed is nonpolar covalent.
1 When Electrons Are Unequally Shared Between Two Atoms In A Bond The Bond Is Said To Be. Both CH_4 and H_2O have only single bonds so each bond has 2 electrons. - 20602642 hanpot64588 hanpot64588 01132021 Chemistry.
The bond only pairs them. Find step-by-step Chemistry solutions and your answer to the following textbook question. Bond length is the distance between.
A covalent bond can be thought of as a shared pair of electrons so there are 2 electrons in each bond. Formation of hydrogen molecules. But in a double bond one atom will share two of its unpaired electrons with two of another electrons unpaired electrons for a total of four.
Indicates the attraction of an atom for shared electrons. How many electrons are shared in a double covalent bond. This type of covalent bond includes four bonding electrons between atoms rather than the usual two bonding electrons that are involved in a single bond.
A double bond is formed when two atoms share two pairs of electrons. It is depicted by two horizontal lines between two atoms in a molecule. For eg in O 2.
Double bonds are made of one pi bond and one sigma bond. Covalent is the same as _________.

How Many Electrons Are Shared In A Double Covalent Bond

Question Video Recalling The Number Of Electrons In A Double Covalent Bond Nagwa
How Many Electrons Are Shared In A Double Bond Quora

Learn The Difference Between Ionic And Covalent Bonds See Examples Of The Two Types Of Ch Ionic And Covalent Bonds Covalent Bonding Covalent Bonding Worksheet
Covalent Bond High Resolution Stock Photography And Images Alamy
How Many Electrons Are Shared In A Double Bond Quora

Single And Multiple Covalent Bonds Article Khan Academy

Single And Multiple Covalent Bonds Article Khan Academy

Single And Multiple Covalent Bonds Article Khan Academy

Covalent Bond Definition Types And Examples
What Is A Double Covalent Bond Quora

Covalent Bond Ck 12 Foundation
When Atoms In A Covalent Bond Share Electrons Is The Sharing Equal Quora

Question Video Recalling The Number Of Electrons In A Triple Covalent Bond Nagwa
How Many Electrons Are Shared In A Double Bond Quora
In A Double Covalent Bond How Many Total Electrons Are Being Shared Quora

Comments
Post a Comment